3. Pressure, Temperature, and Density
Pressure
When we talk about pressure in the ocean, we are referring to hydrostatic pressure, which is a result of the weight of the water column pressing down on an object due to gravity. The deeper you go, the more water that is above you, and the greater the weight (and thus pressure) of that water. At the surface we experience one atmosphere of pressure (1 atm = 101.3 kPa) due to the weight of the atmosphere above us. As you descend into the ocean, pressure increases linearly with depth; there is an increase in pressure of 1 atm for every 10 m increase in depth. So at 1000 m depth the pressure would be 101 atm (100 atm of pressure due to the 1000 m depth, plus the 1 atm that is present at the surface). This leads to very high pressure in the deeper parts of the ocean; if you consider that the average depth of the ocean is about 3800 m, the pressure at that depth would be 381 times greater than the pressure at the surface.
There are several important consequences of high pressure at depth. First, due to Boyle’s Law, which states that the volume of a gas is inversely related to pressure, high pressure will act to compress air spaces, such as the lungs of a diving animal (or person), or the space inside a submarine. Submarines and submersibles must therefore have very strong hulls to resist this compression at extreme depths. Second, Henry’s Law provides that at higher pressures a fluid will contain more dissolved gas. This means that deeper, high pressure water may contain more dissolved gases than surface water.
This also has implications for human divers. According to Henry’s Law, if you increase pressure, you increase the amount of gas that can dissolve in a fluid (such as blood). Conversely, when you reduce pressure, the fluid holds less dissolved gas, and the excess gas will leave the solution, often in the form of bubbles. This is what happens when you open a bottle of a carbonated beverage. The contents in the bottle are sealed under pressure, and as you open the bottle, you release the pressure, and the fluid can no longer hold all of the CO2 that was dissolved in it, so the CO2 escapes, forming bubbles. Decompression sickness, or “the bends” occurs in SCUBA divers if they ascend too quickly after breathing compressed air. A slow ascent allows this excess gas to be removed from the blood and exhaled, but if the diver ascends too quickly, these gases will come out of solution and form bubbles in the blood that congregate near the joints, causing intense pain and perhaps death.
Additional links for more information
- How can deep-diving marine mammals avoid “the bends”?: http://www.oneworldoneocean.com/blog/entry/ocean-stemulation-how-marine-mammals-avoid-the-bends
Temperature
Generally ocean temperatures range from about -2o to 30o C (28-86o F). The warmest water tends to be surface water in low latitude regions, while the surface water at the poles is obviously much colder (Figure 4.3.1). Note that at equivalent latitudes, water on the eastern side of the ocean basins is colder than the water on the western side. This has to do with the pattern of surface currents. Even though surface water can be quite warm, most of the water in the oceans is deeper, colder water, so that the average temperature of the entire ocean is about 4o C, which is roughly the temperature inside your refrigerator.
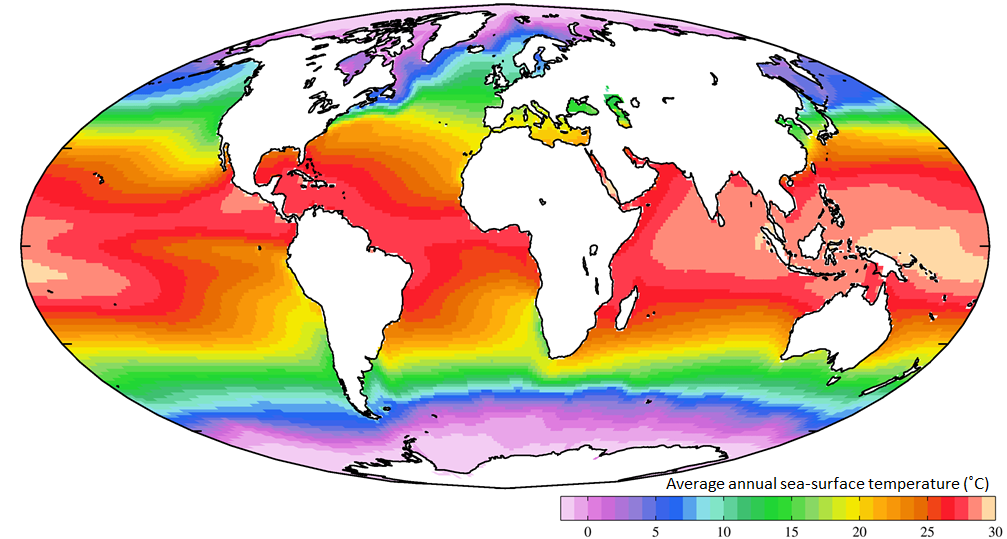
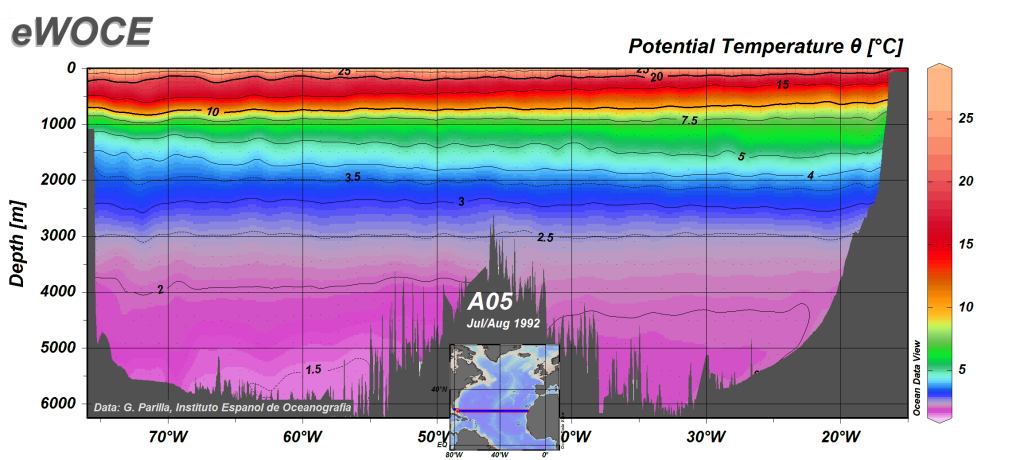
A typical temperature profile for open ocean, mid-latitude water is shown in Figure 4.3.2. Water is warmest at the surface, as it is warmed by the sun, and the sun’s rays can only penetrate depths less than 1000 m. Since the surface water is warmer it is also less dense than the deep water, so it remains at the surface where it can be warmed even more. Temperature is fairly constant in the upper 100-200 m in what is called the mixed layer. The mixed layer results from surface winds, waves, and currents that mix the upper water and distribute the heat throughout this layer. Below the mixed layer there is a rapid decline in temperature over a fairly narrow increase in depth. This is called thethermocline. Below the thermocline the deep ocean temperature is fairly constant at about 2o C, continuing down to the bottom. There is little temperature change in the deep ocean, as it is far removed from significant heat sources, making it one of the most thermally stable regions on earth. Temperature may fluctuate by less than half a degree per year in the deep ocean (Figure 4.3.3).
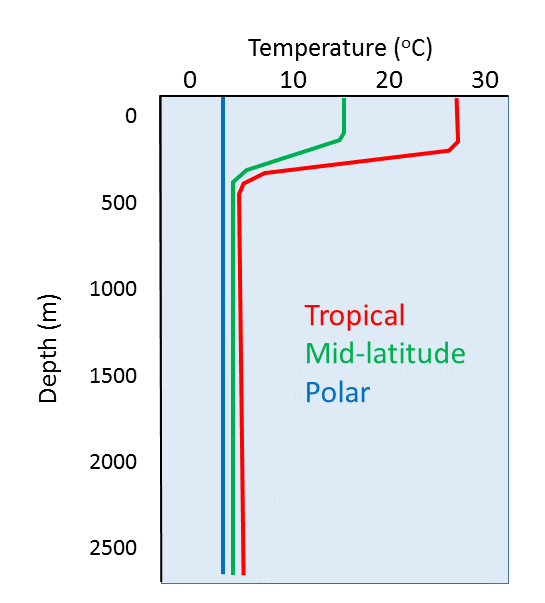
Temperature profiles vary at different latitudes, as the surface water is warmer near the equator and colder at the poles. In low latitude tropical regions the sea surface is much warmer, leading to a highly pronounced thermocline (Figure 4.3.4). Additionally, there is not much seasonal change in surface temperature in tropical regions, so there is little seasonal change in the profiles. In high latitude (polar) regions, there is little difference between the surface temperature and the deep water temperature, and temperature is fairly constant (and cold) at all depths. Polar waters therefore lack a strong thermocline, and as with tropical water, there is little seasonal change in temperatures. Mid-latitude temperate regions show greater seasonal fluctuations in surface temperature than the poles or the tropics; an 8-15o C difference from summer to winter in temperate zones, compared to only ~2o C in polar and tropical areas. In temperate regions, the surface water is much warmer in the summer and the thermocline is more pronounced compared to the winter months. But in the winter the thermocline is deeper at mid-latitudes than it is in the summer. This is because winter storms churn up the surface water more than occurs in the summer, creating a deeper mixed layer and thus a deeper thermocline (Figure 4.3.5).
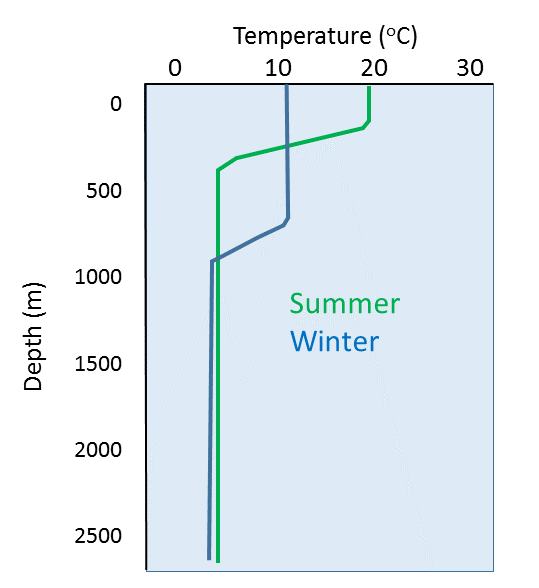
Due to the high heat capacity of water, daily fluctuations in ocean temperature are fairly insignificant.
Density
Density refers to the amount of mass per unit volume, such as grams per cubic centimeter (g/cm3). The density of fresh water is 1 g/cm3 at 4o C, but the addition of salts and other dissolved substances increases surface seawater density to between 1.02 and 1.03 g/cm3. The density of seawater can be increased by reducing its temperature, increasing its salinity, or increasing the pressure. Pressure has the least impact on density as water is fairly incompressible, so pressure effects are not very significant except at extreme depths. However, if not for the slight compression of water due to pressure, sea level would be approximately 50 m higher than it is today! That leaves temperature and salinity as the primary factors determining density, and of these, temperature has the greatest impact (Figure 4.3.6).
Since temperature has the greatest effect on density, density profiles are usually mirror images of temperature profiles (Figure 4.3.7). Density is lowest at the surface, where the water is the warmest. As depth increases, there is a region of rapidly increasing density with increasing depth, which is called the pycnocline. The pycnocline coincides with the thermocline, as it is the sudden decrease in temperature that leads to the increase in density. Below the pycnocline, density may be fairly constant (as is temperature), or it may continue to increase slightly towards the bottom.
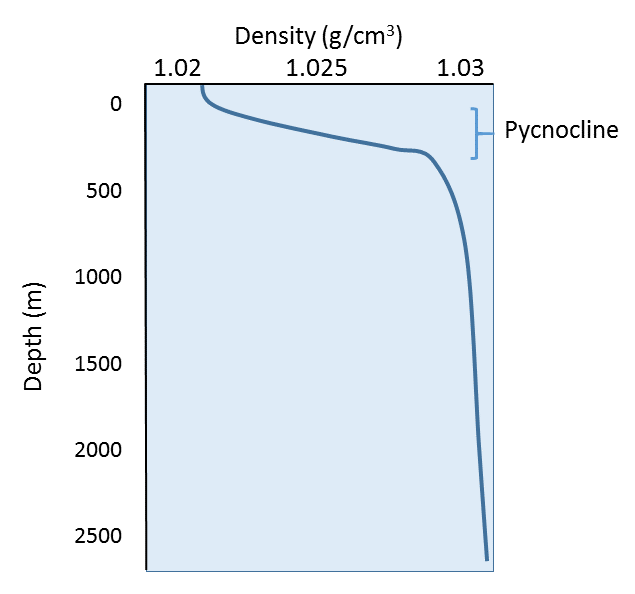
The profile above represents a stable state, or a high degree of stratification, where the warm, low density layer sits atop the colder, denser layer. If denser water happened to form at the surface, the water masses would be unstable, and the denser water would sink to the bottom, to be replaced by less dense water at the surface. This vertical movement of water masses based on density (as determined by temperature and salinity) is referred to as thermohaline circulation. By creating a stratified water column, the thermocline and pycnocline together create a barrier that prevents mixing between the warmer, less dense surface water and the colder, denser bottom water. In this way, nutrient-rich deep water may be prevented from coming to the surface to support primary production.
As with temperature, there are also latitudinal differences in density. In the tropics the surface water is warm and low density, and there is a pronounced thermocline separating it from the colder, denser deep water. As stated above, this stratification prevents nutrient-rich water from reaching the surface and as a result tropical regions often have low productivity. In the high latitudes the water is uniformly cold at all depths, so there is little density stratification. The lack of a pycnocline (or a thermocline) allows cold, nutrient-rich deep water to more easily mix with the surface water, leading to higher primary production in polar regions.

