Western blot
Summary
Western blots assess how much of a particular protein is present in a sample. The thicker or darker the band, the more of the protein is present in the sample.
Also known as: Western, immunoblot
Samples needed: Cell or tissue lysates or other solutions to be assessed for levels of a particular protein
Controls:
To be able to compare quantity of a particular protein across lanes, it is necessary to make sure the same total amount of protein has been loaded in each lane. Therefore, a loading control is required. Usually this takes one of two forms. First, the membrane can be stained for total protein before immunoblotting with a protein-binding dye like Ponceau stain (red). Alternatively, the membrane can be immunoblotted for a “housekeeping” protein like GAPDH, actin, tubulin, etc.
Method:
To perform a Western blot, samples are subject to gel electrophoresis (link to entry). Then, proteins are transferred from the gel to a paper-like membrane using an electric current. The membrane is then blocked, often with milk protein, to discourage any non-specific binding of antibodies to proteins on the membrane. Next, the membrane is incubated in a primary antibody, which specifically binds to the protein of interest. Then, the membrane is incubated in a secondary antibody, which binds to the primary antibody based on what species the primary antibody was raised in. (For instance, the secondary antibody might be something like anti-rabbit IgG.) The secondary antibody is modified with a molecule that allows for easy detection of bands. Common modes of detection are fluorescence or luminescence. Finally, the Western blot is visualized and photographed, using a method appropriate for the secondary antibody used.
Interpretation:
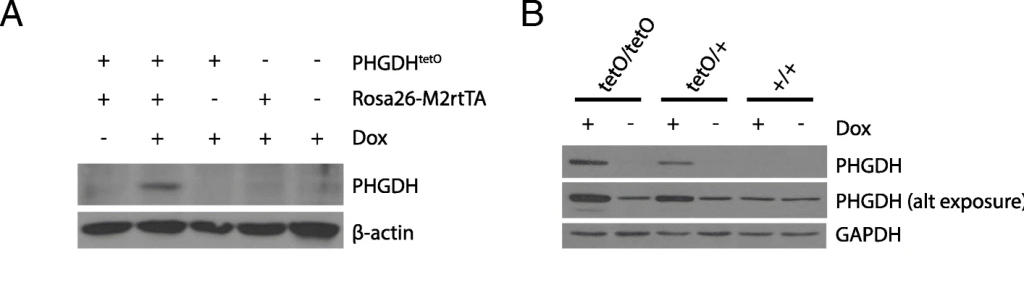
Figure 1. Two Western blots assessing PHGDH expression. Relevant section of caption for published figure reads: “a Western blot analysis to assess PHGDH expression in liver lysates from mice harboring the indicated alleles that were exposed to a doxycycline containing diet (Dox) or a control diet for 5 days. β-actin expression was also assessed as a loading control. b Western blot analysis to assess PHGDH expression in MEFs derived from mice with the Rosa26-M2rtTA allele and the indicated number of PHGDHtetO (tetO) alleles that were cultured in media with or without doxycycline (Dox) as for 72 h as indicated. Both a light and dark exposure (alt exposure) are shown, as is GAPDH expression as a loading control.” “Figure 1” by Katherine R. Mattaini, Mark R. Sullivan, Allison N. Lau, Brian P. Fiske, Roderick T. Bronson & Matthew G. Vander Heiden[1]. [Image description]
Figure 1 is from a study of a newly-generated genetically engineered mouse. The mouse conditionally expresses the protein PHGDH. PHGDH protein should be produced only if a) the PHGDHtetO allele is present, b) an rtTA allele is present, and c) the mouse is administered the compound doxycycline (dox). In Figure 1A, the reader sees a band in the PHGDH blot only when all three criteria are met. In all lanes, the β-actin band is a similar weight and thickness, showing that the same amount of protein was loaded in each lane. (In lane 3, there is a semi-circle missing from the band, indicating that there was likely a bubble when the transfer from the gel to the membrane was taking place.)
In Figure 1B, the authors were testing MEFs generated from the new mice, and testing that they produced PHGDH protein in the expected way. Three genotypes of MEF are shown: those with two PHGDHtetO alleles, those with one, and those with none. In the top panel, the reader can see that the most PHGDH protein is present only with a PHGDHtetO allele and dox. The darker band in the first lane also indicates that more PHGDH protein is present with two PHGDHtetO alleles than one. The panel below that shows PHGDH blotting with a longer exposure, showing that there is some PHGDH expressed in the MEFs at all times, but more is present when expression from the transgene is induced. The final panel shows that the same amount of GAPDH is present in all lanes, indicating that the same amount of total protein was loaded in each lane.
Special cases:
Western blot for post-translational modifications: In certain fields, it is common to perform Western blots to assess how much of a post-translationally modified protein is present in a sample, for example, phosphorylation of protein X at a particular site. In this instance, it is necessary to blot for both phosphorylated protein X and total protein X. Otherwise, if you see an increase in levels of phospho-protein X, it is unclear whether that means more protein X overall or the same amount of protein X but more of it phosphorylated.
Image Descriptions
Figure 1 image description: Two Western blots. In the first blot, PHGDH expression only occurs when PHGDHtetO, Rosa26-M2rtTA, and dox are all present. β-actin loading control is consistent. In the second blot, at a low exposure PHGDH is visible in tetO/tetO and tetO/+ genotypes only when dox is present, and not in +/+ genotype. The band is slightly darker in tetO/tetO than tetO/+. At a higher exposure, PHGDH is present in all lanes, but much more prominent in the tetO/tetO and tetO/+ lanes with dox. GAPDH loading control is consistent. [Return to Figure 1]
- Mattaini, K. R., M. R. Sullivan, A. N. Lau, B. P. Fiske, R. T. Bronson, and M. G. Vander Heiden. 2019. Increased PHGDH expression promotes aberrant melanin accumulation. BMC Cancer 19:723. ↵
Proteins are one class of biological macromolecules. They are made of amino acid building blocks and have many biological functions including catalysis, structure, transport, signaling, and others.
The entire contents of a cell, released by breaking open the plasma membrane
A protein that binds very specifically to a protein of interest, normally produced by an organism's adaptive immune system to protect against pathogens, but also used in a variety of biotechnological applications
Specific variant (sequence) of a gene
Mouse embryonic fibroblasts, for growing in cell culture
A gene that has been transferred from one organism to another; for instance, a human gene expressed in a genetically engineered mouse