Chapter 9. Cell Communication

Chapter Outline
Type your examples here.
- 9.1 Signaling Molecules and Cellular Receptors
- 9.2 Propagation of the Signal
- 9.3 Response to the Signal
Introduction
Imagine what life would be like if you and the people around you could not communicate. You would not be able to express your wishes to others, nor could you ask questions to find out more about your environment. Social organization is dependent on communication between the individuals that comprise that society.
As with people, it is vital for individual cells to be able to interact with their environment and with each other. This is true whether a cell is growing by itself in a pond or is one of many cells that form a larger organism. In order to properly respond to external stimuli, cells have developed complex mechanisms of communication so that they can receive a message, transfer the information across the plasma membrane, and then produce changes within the cell in response to the message.
In multicellular organisms, cells constantly send and receive chemical messages to coordinate the actions of other organs, tissues, and cells. The ability to send messages quickly and efficiently enables cells to coordinate and fine-tune their functions. While the necessity for cellular communication in larger organisms seems obvious, even single-celled organisms communicate with each other. Yeast cells signal each other to aid mating. Some forms of bacteria coordinate their actions in order to form large complexes called biofilms or to organize the production of toxins to remove competing organisms. The ability of cells to communicate through chemical signals originated in single cells and was essential for the evolution of multicellular organisms. Efficient, error-free communication is vital for all life.
9.1 | Signaling Molecules and Cellular Receptors
Learning Objectives
By the end of this section, you will be able to:
- Describe four types of signaling found in multicellular organisms.
- Compare internal receptors with cell-surface receptors.
- Recognize the relationship between a ligand’s chemistry and its mechanism of action.
There are two kinds of communication in the world of living cells. Communication between cells is called intercellular signaling, and communication within a cell is called intracellular signaling. An easy way to remember the distinction is by understanding that the prefix inter- means “between” (an interstate highway crosses between states) and intra- means “inside” (an IV means intravenous or “inside the vein”).
Chemical signals are released by a signaling cell and received by a target cell. Target cells have proteins called receptors, which bind to signaling molecules and cause a response. Signaling molecules that bind to receptors are called ligands. Ligands and receptors are specific for each other; a receptor will typically bind only to its specific ligand. However, there are different types of signaling.
9.1.1 Forms of Signaling
There are four categories of chemical signaling found in multicellular organisms: autocrine signaling, paracrine signaling, endocrine signaling, and direct signaling across gap junctions (Figure 9.2). The main difference between the different categories of signaling is the distance that the signal travels to reach the target cell.
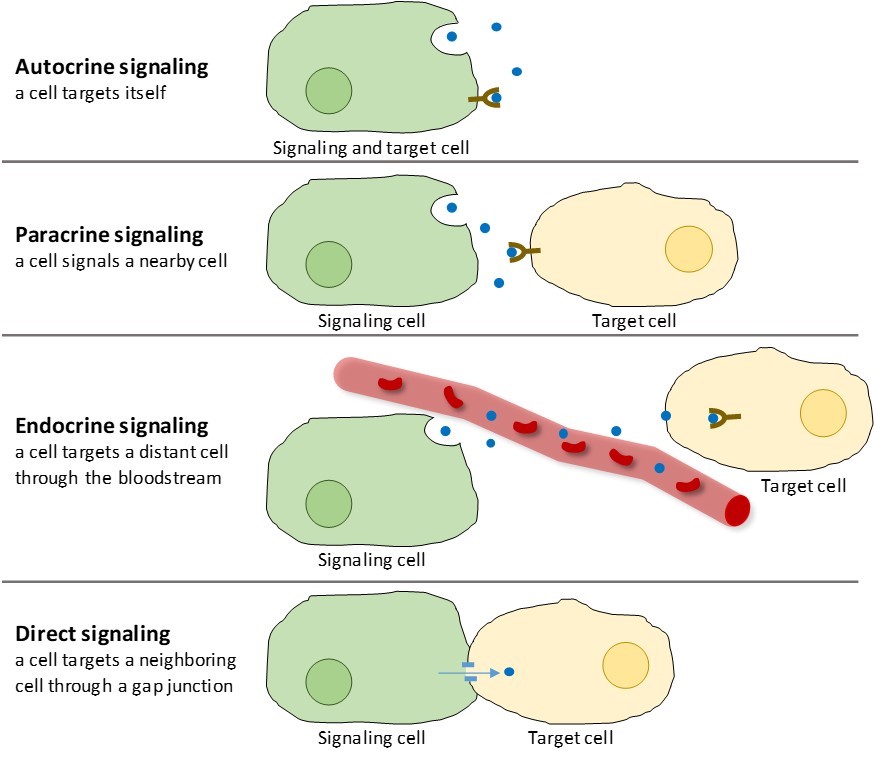
Paracrine Signaling
Signals that act locally between cells that are close together are called paracrine signals. Paracrine signals move by diffusion through the extracellular matrix (Figure 9.2). These types of signals usually elicit quick responses that last only a short amount of time. In order to keep the response localized, paracrine ligands are usually quickly degraded by enzymes or removed by neighboring cells. Removing the signals reestablishes the concentration gradient for the signal molecule, allowing them to quickly diffuse through the intracellular space if released again.
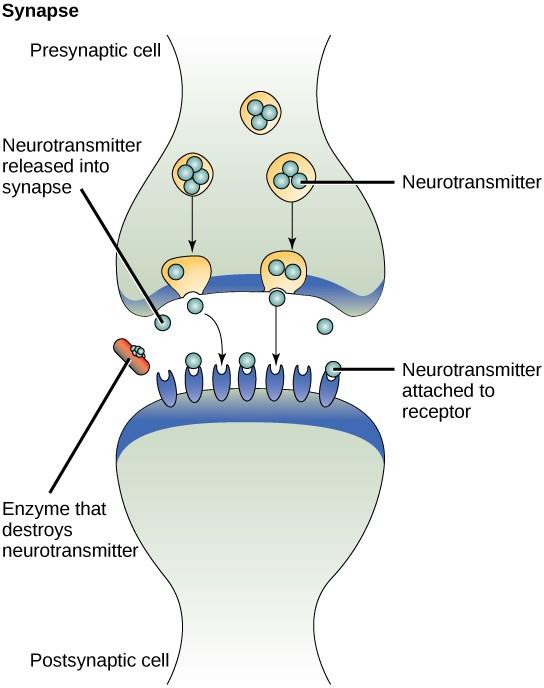
One example of paracrine signaling is the transfer of signals between nerve cells. The tiny space between nerve cells where signal transmission occurs is called a synapse. Signals are propagated along nerve cells by fast-moving electrical impulses. When these impulses reach the end of one nerve cell, chemical ligands called neurotransmitters are released into the synapse by the presynaptic cell (the cell emitting the signal). The neurotransmitters diffuse across the synapse (Figure 9.3). The small distance between nerve cells allows the signal to travel quickly, which enables an immediate response, such as, “take your hand off the stove!” When the neurotransmitter binds the receptor on the surface of the postsynaptic cell, the next electrical impulse is launched. The neurotransmitters are degraded quickly or are reabsorbed by the presynaptic cell so that the recipient nerve cell can recover quickly and be prepared to respond rapidly to the next synaptic signal.
Autocrine Signaling
When a cell responds to its own signaling molecule, it is called autocrine signaling (auto = “self”). Autocrine signaling often occurs with other types of signaling. For example, when a paracrine signal is released, the signaling cell may respond to the signal along with its neighbors (Figure 9.2).
Autocrine signaling often occurs during early development of an organism to ensure that cells develop into the correct tissues. Autocrine signaling also regulates pain sensation and inflammatory responses. Further, if a cell is infected with a virus, the cell can signal itself to undergo programmed cell death, killing the virus in the process.
Endocrine Signaling
Signals from distant cells are called endocrine signals, and they originate from endocrine cells. (In the body, many endocrine cells are located in endocrine glands, such as the thyroid gland, the hypothalamus, and the pituitary gland.) These types of signals usually produce a slower response but have a longer-lasting effect. The ligands released in endocrine signaling are called hormones, signaling molecules that are produced in one part of the body but affect other body regions some distance away (Figure 9.2).
Hormones travel the large distances between endocrine cells and their target cells via the bloodstream, which is a relatively slow way to move throughout the body. Because of their form of transport, hormones get diluted and are present in low concentrations when they act on their target cells. This is different from paracrine signaling, in which local concentrations of signaling molecules can be very high.
Direct Signaling
Gap junctions in animals and plasmodesmata in plants are connections between the plasma membranes of neighboring cells. These water-filled channels allow small signaling molecules to diffuse between the two cells. Small molecules, such as calcium ions (Ca2+), are able to move between cells, but large molecules like proteins and DNA cannot fit through the channels. The specificity of the channels ensures that the cells remain independent but can quickly and easily transmit signals. Direct signaling allows a group of cells to coordinate their response to a signal that only one of them may have received. In plants, plasmodesmata are ubiquitous, making the entire plant into a giant communication network.
9.1.2 Types of Receptors
Receptors are protein molecules in the target cell or on its surface that bind to ligands. There are two types of receptors, internal receptors and cell-surface receptors.
Internal receptors
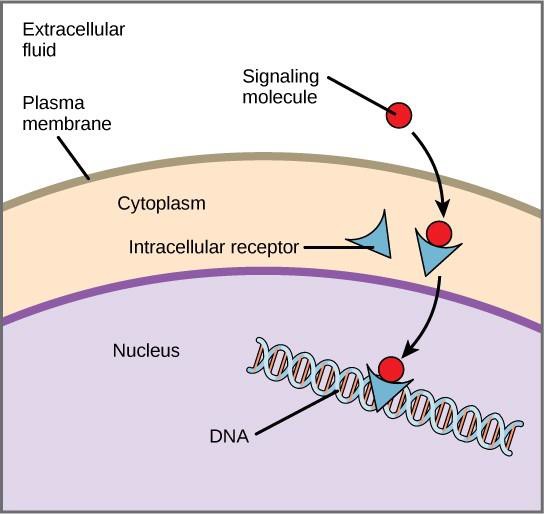
Internal receptors, also known as intracellular or cytoplasmic receptors, are found in the cytoplasm of target cells and respond to hydrophobic ligand molecules that are able to travel across the plasma membrane. Once inside the cell, many of these molecules bind to proteins that act as regulators of mRNA synthesis (transcription) to mediate gene expression.
Gene expression is the cellular process of transforming the information in a cell’s DNA into a sequence of amino acids, which ultimately forms a protein. When the ligand binds to the internal receptor, a conformational change is triggered that exposes a DNA-binding site on the receptor protein. The ligand-receptor complex moves into the nucleus, then binds to specific regulatory regions of the chromosomal DNA and promotes the initiation of transcription (Figure 9.4). Transcription is the process of copying the information in a cell’s DNA into a special form of RNA called messenger RNA (mRNA); the cell uses information in the mRNA to link specific amino acids in the correct order, producing a protein. Thus, when a ligand binds to an internal receptor, it can directly influence gene expression in the target cell.
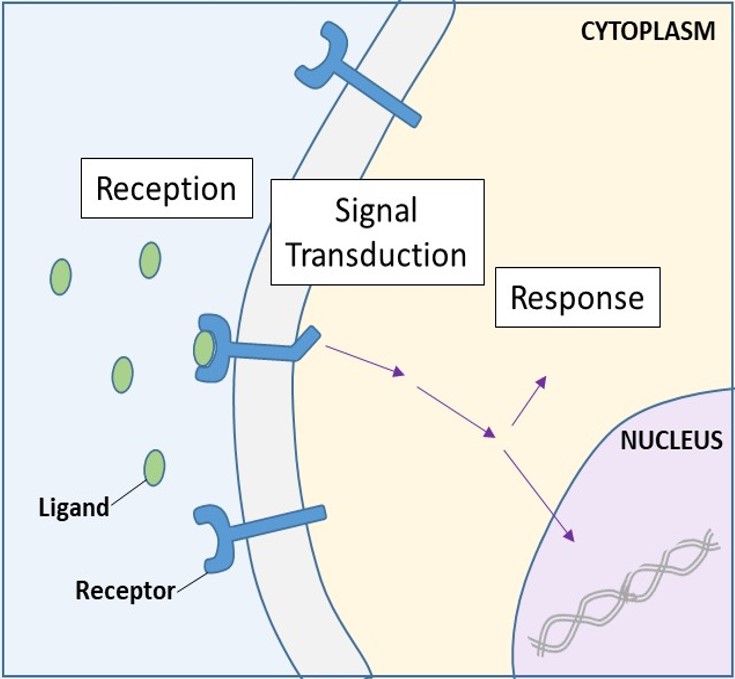
Cell-Surface Receptors
Cell-surface receptors, also known as transmembrane receptors, are integral proteins that bind to external signaling molecules. These receptors span the plasma membrane and perform signal transduction, in which an extracellular signal is converted into an intercellular signal. (Figure 9.5). Because cell-surface receptor proteins are fundamental to normal cell functioning, it should come as no surprise that a malfunction in any one of these proteins could have severe consequences. Errors in the protein structures of certain receptor molecules have been shown to play a role in hypertension (high blood pressure), asthma, heart disease, and cancer.
Each cell-surface receptor has three main components: an external ligand-binding domain, or extracellular domain; a hydrophobic membrane-spanning region; and an intracellular domain. Cell-surface receptors are involved in most of the signaling in multicellular organisms. There are three general categories of cell-surface receptors: enzyme-linked receptors, ion channel-linked receptors, and G-protein-linked receptors.
Enzyme-linked receptors are cell-surface receptors with intracellular domains that are associated with an enzyme. In some cases, the intracellular domain of the receptor itself is an enzyme. Other enzyme-linked receptors have a small intracellular domain that interacts directly with an enzyme. Enzyme-linked receptors normally have large extracellular and intracellular domains, but the membrane-spanning region consists of a single alpha-helix in the peptide strand.
When a ligand binds to the extracellular domain of an enzyme-linked receptor, a signal is transferred through the membrane, activating the enzyme. Activation of the enzyme sets off a chain of events within the cell that eventually leads to a response.
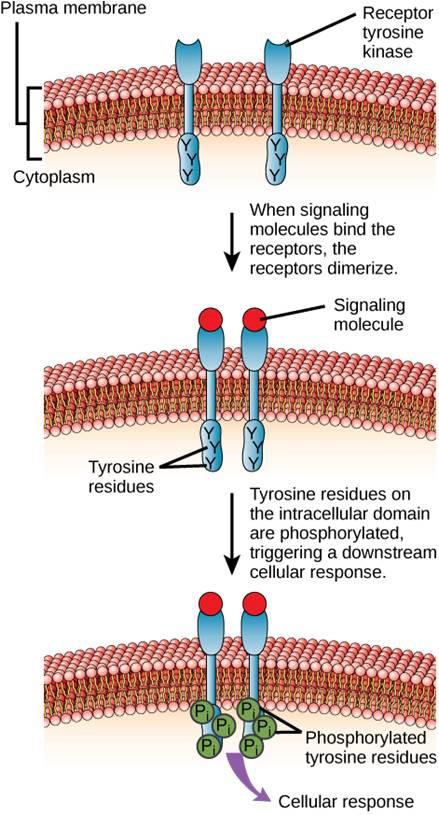
One example of an enzyme-linked receptor is the tyrosine kinase receptor (Figure 9.6). A kinase is an enzyme that transfers phosphate groups from ATP to another protein. The tyrosine kinase receptor transfers phosphate groups to tyrosine molecules. First, signaling molecules bind to the extracellular domain of two nearby tyrosine kinase receptors. The two neighboring receptors then bond together, or dimerize. Phosphates are then added to tyrosine residues on the intracellular domain of the receptors (phosphorylation). The phosphorylated residues can then transmit the signal to the next messenger within the cytoplasm.
Epidermal growth factor receptors are an example of receptor tyrosine kinases that follows this mode of signaling. Defects in ErbB signaling in this family can lead to neuromuscular diseases such as multiple sclerosis and Alzheimer’s disease.
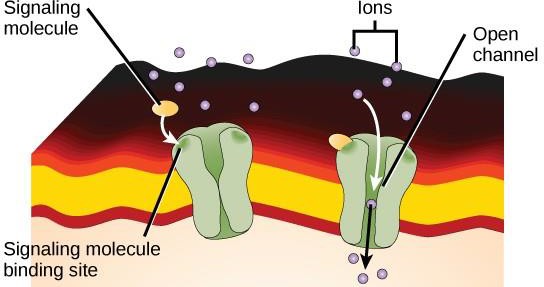
Ion channel-linked receptors bind to a ligand and open a channel through the membrane that allows specific ions to pass through. This type of cell-surface receptor has an extensive membrane-spanning region with hydrophobic amino acids. Conversely, the amino acids that line the inside of the channel are hydrophilic to allow for the passage of ions. When a ligand binds to the extracellular region of the channel, there is a conformational change in the protein’s structure that allows ions such as sodium, calcium, magnesium, or hydrogen to pass through (Figure 9.7).
G-protein-linked receptors bind to a ligand and activate an associated G-protein. The activated G- protein then interacts with a nearby membrane protein, which may be an ion channel or an enzyme (Figure 9.8). All G-protein-linked receptors have seven transmembrane domains, but each receptor has a specific extracellular domain and G-protein-binding site.
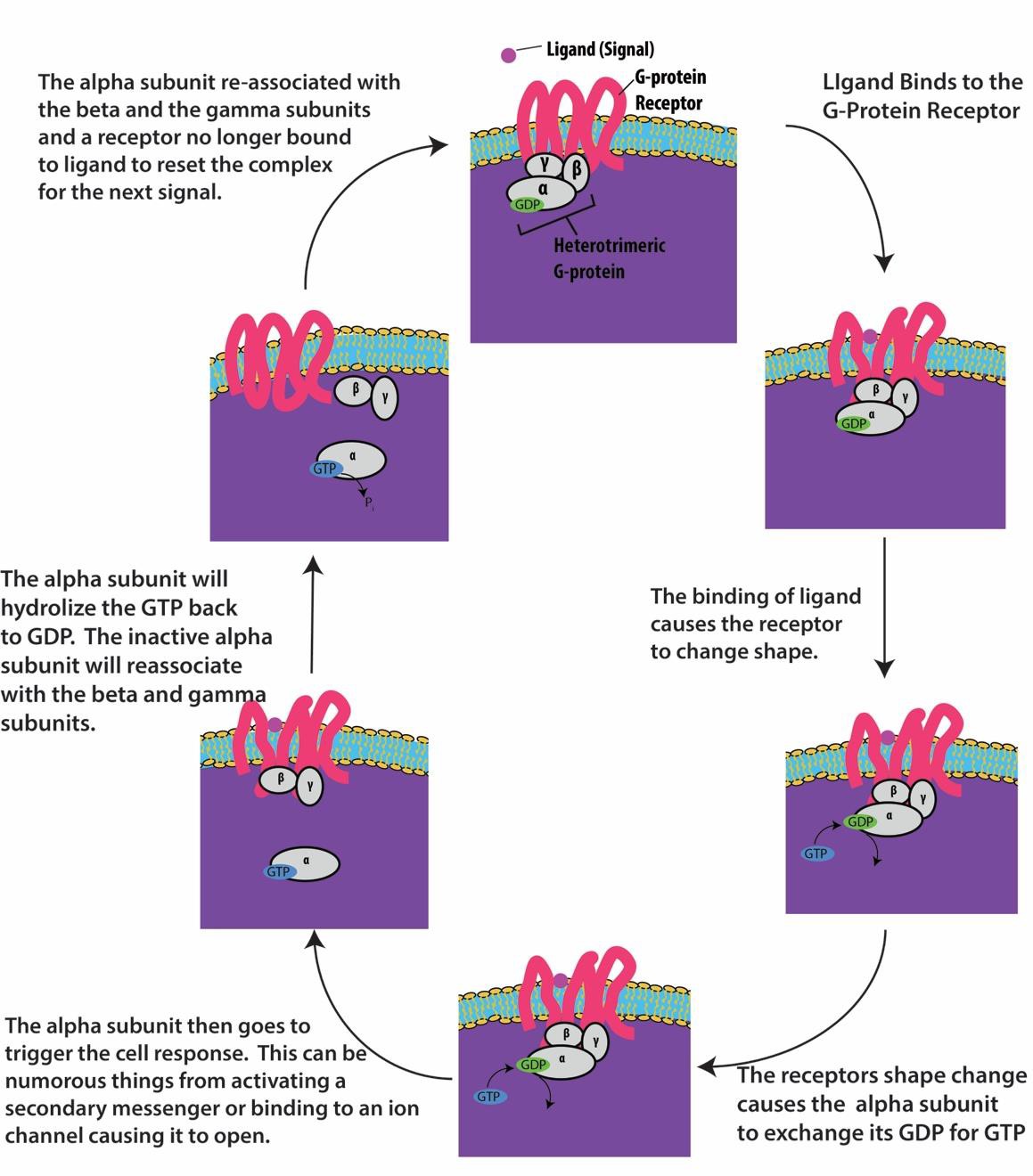
Cell signaling using G-protein-linked receptors occurs as a cycle. Once the ligand binds to the receptor, the resultant shape change activates the G-protein, which releases GDP and picks up GTP. The subunits of the G-protein then split into α and βγ subunits. One or both of these G-protein fragments may be able to activate other proteins in the cell. After a while, the GTP on the active α subunit of the G-protein is hydrolyzed to GDP and the βγ subunit is deactivated. The subunits re-associate to form the inactive G-protein and the cycle begins again (Figure 9.8).
G-protein linked receptors are used in many physiological processes including those for vision transduction, taste, and regulation of immune system and inflammation.

Concept check
HER2 is a receptor tyrosine kinase. In 30 percent of human breast cancers, HER2 is permanently activated, resulting in unregulated cell division. Lapatinib, a drug used to treat breast cancer, inhibits the process by which the receptor phosphorylates itself, thus reducing tumor growth by 50 percent. Which of the following steps would be inhibited by Lapatinib?
- Signaling molecule binding, dimerization, and the downstream cellular response
- Dimerization, and the downstream cellular response
- The downstream cellular response
- Phosphatase activity, dimerization, and the downstream cellular response
9.1.3 Signaling Molecules
Produced by signaling cells, ligands are chemical signals that travel to target cells and cause a response. The types of molecules that serve as ligands are incredibly varied and range from small proteins to small ions. Ligands are categorized as either small hydrophobic ligands, which can cross plasma membranes, or water-soluble ligands, which cannot.
Small Hydrophobic Ligands
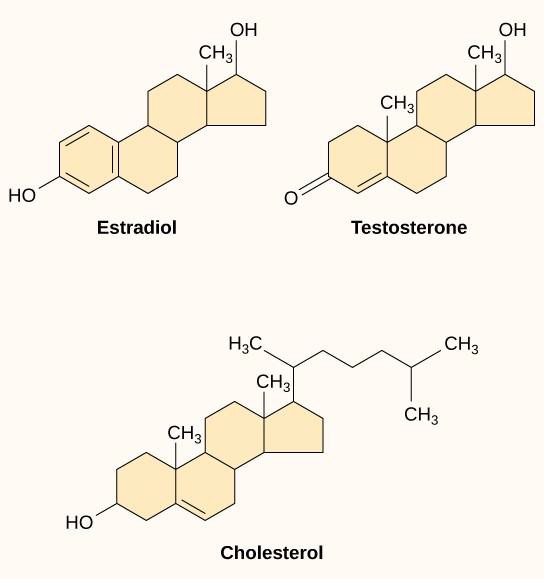
Small hydrophobic ligands, also called lipid-soluble ligands, can directly diffuse through the plasma membrane and interact with internal receptors. Important members of this class of ligands are the steroid hormones. Steroids are lipids that have a hydrocarbon skeleton with four fused rings; different steroids have different functional groups attached to the carbon skeleton. Steroid hormones include the female sex hormone estradiol, which is a type of estrogen; the male sex hormone testosterone; and cholesterol, which is an important structural component of biological membranes and a precursor of steroid hormones (Figure 9.10). Other hydrophobic hormones include thyroid hormones and vitamin D. In order to be soluble in blood, hydrophobic ligands must bind to carrier proteins while they are being transported through the bloodstream.
Water-Soluble Ligands
Since water-soluble ligands are polar, they cannot pass through the plasma membrane unaided. Sometimes they are too large to pass through the membrane at all. Instead, most water-soluble ligands bind to the extracellular domain of cell-surface receptors (see Figure 9.5). This group of ligands is quite diverse and includes small molecules, peptides, and proteins.
9.2 | Propagation of the Signal
Learning Objectives
By the end of this section, you will be able to:
- Explain how the binding of a ligand initiates signal transduction throughout a cell.
- Recognize that intracellular signals are transmitted by the role of phosphorylation or second messengers.
Once a water-soluble ligand binds to its receptor, the signal is transmitted through the membrane and into the cytoplasm. Continuation of a signal in this manner is called signal transduction (Figure 9.5). Signal transduction only occurs with cell-surface receptors since internal receptors are able to enter the cell.
When a ligand binds to its receptor, conformational changes occur that affect the receptor’s intracellular domain. These conformational changes lead to activation of the intracellular domain or its associated proteins. In some cases, binding of the ligand causes dimerization of the receptor, which means that two receptors bind to each other to form a stable complex called a dimer. A dimer is a chemical compound formed when two molecules (often identical) join together. The binding of the receptors in this manner enables their intracellular domains to come into close contact and activate each other.
9.2.1 Signaling Pathways and Signal Amplification
Although signaling molecules are often found at very low concentrations, they may produce profound effects. After the ligand binds to the cell-surface receptor, the activation of the receptor’s intracellular components sets off a chain of events that is called a signaling pathway or a signaling cascade. In a signaling pathway, second messengers, enzymes, and/or activated proteins activate other proteins or messengers (Figure 9.11). Each member of the pathway can activate thousands of the next member of the pathway in a process called signal amplification. Since the signal is amplified at each step, a very large response can be generated from a single receptor binding a ligand.
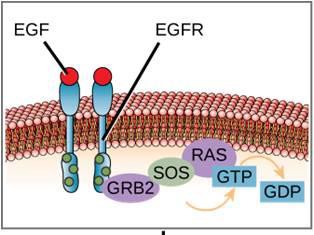
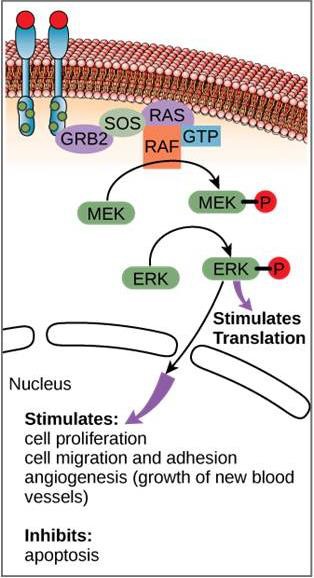
An example of a signaling pathway is shown is Figure 9.11. Epidermal growth factor (EGF) is a signaling molecule that is involved in the regulation of cell growth, wound healing, and tissue repair. The receptor for EGF (EGFR) is a tyrosine kinase. An activated kinase phosphorylates and activates many downstream molecules. When EGF binds to EGFR, a cascade of downstream phosphorylation events signals the cell to grow and divide. If EGFR is activated at inappropriate times, uncontrolled cell growth (cancer) may occur.
Concept Check
In certain cancers, the GTPase activity of the RAS G-protein is inhibited. This means that the RAS protein can no longer hydrolyze GTP into GDP. What effect would this have on downstream cellular events?
9.2.2 Methods of Intracellular Signaling
The induction of a signaling pathway depends on the modification of a cellular component by an enzyme. There are numerous enzymatic modifications that can occur to activate the next component of the pathway. The following are some of the more common events in intracellular signaling.
Phosphorylation
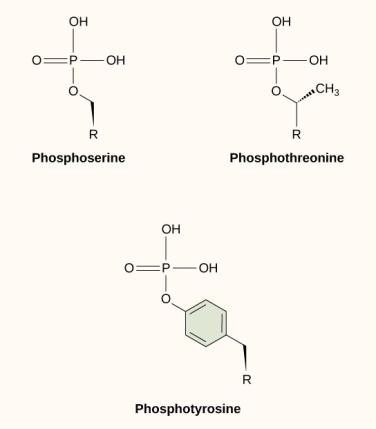
One of the most common chemical modifications that occurs in signaling pathways is the addition of a phosphate group to a molecule in a process called phosphorylation. The phosphate can be added to a nucleotide such as GMP to form GDP or GTP. Phosphates are also often added to serine, threonine, and tyrosine residues of proteins, where they replace the hydroxyl group of the amino acid (Figure 9.12). The transfer of the phosphate is catalyzed by an enzyme called a kinase. Phosphorylation may activate or inactivate enzymes, and the reversal of phosphorylation, dephosphorylation, will reverse the effect.
Second Messengers
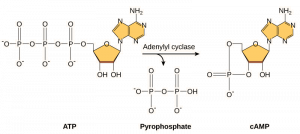
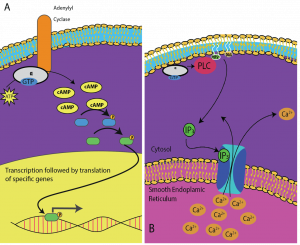
Second messengers are small molecules that propagate a signal after it has been initiated by the binding of the signaling molecule to the receptor. These molecules help to spread a signal through the cytoplasm by altering the behavior of certain cellular proteins. A second messenger utilized by many different cell types is cyclic AMP (cAMP). Cyclic AMP is synthesized by the enzyme adenylyl cyclase from ATP (Figure 9.13). The main role of cAMP in cells is to bind to and activate an enzyme called cAMP-dependent kinase (A-kinase). A-kinase regulates many vital metabolic pathways: It phosphorylates serine and threonine residues of its target proteins, activating them in the process. A-kinase is found in many different types of cells, and the target proteins in each kind of cell are different. Another secondary messenger is Ca2+which can be released to flood the cell.
Different cells respond differently to cAMP. In Figure 9.14, the alpha subunit from a G-protein receptor is shown activating two different types of signaling. In the first image, cAMP is produced by the enzyme adenylate cyclase when activated by the alpha subunit. cAMP then activates other proteins that affect gene transcription. In the second image, the alpha subunit from the G-protein triggers a cascade that releases Ca2+ from the smooth endoplasmic reticulum. In this case Ca2+ is the secondary messenger that causes the cellular response.
9.3 | Response to the Signal
Learning Objectives
By the end of this section, you will be able to:
- Recognize that signaling pathways direct protein expression, cellular metabolism, and cell growth.
- Recognize the role of apoptosis in the development and maintenance of a healthy organism.
Using signal transduction pathways, receptors in the plasma membrane produce a variety of effects on the cell. Inside the cell, ligands bind to their internal receptors, allowing them to directly affect the cell’s DNA and protein-producing machinery. The results of signaling pathways are extremely varied and depend on the type of cell involved as well as the external and internal conditions. A small sampling of responses is described below.
9.3.1 Responses to the Signaling Pathway
Gene Expression
Some signal transduction pathways regulate the transcription of RNA. Others regulate the translation of proteins.
Increase in Cellular Metabolism
The activation of β-adrenergic receptors in muscle cells by adrenaline leads to an increase in cyclic AMP inside the cell. Adrenaline is a hormone produced by the adrenal gland that readies the body for short-term emergencies.
Cell Growth
Cell signaling pathways also play a major role in cell division. Cells do not normally divide unless they are stimulated by signals from other cells. The ligands that promote cell growth are called growth factors. Most growth factors bind to cell-surface receptors that are linked to tyrosine kinases.
Cell Death
When a cell is damaged, superfluous, or potentially dangerous to an organism, a cell can initiate a mechanism to trigger programmed cell death, or apoptosis. Apoptosis allows a cell to die in a controlled manner that prevents the release of potentially damaging molecules from inside the cell. However, in some cases, such as a viral infection or uncontrolled cell division due to cancer, the cell’s normal checks and balances fail. External signaling can also initiate apoptosis. For example, most normal animal cells have receptors that interact with the extracellular matrix, a network of glycoproteins that provides structural support for animal cells. The binding of cellular receptors to the extracellular matrix initiates a signaling cascade within the cell. However, if the cell moves away from the extracellular matrix, the signaling ceases, and the cell undergoes apoptosis. This system helps prevent cells from traveling through the body and proliferating out of control, as happens with tumor cells that metastasize.
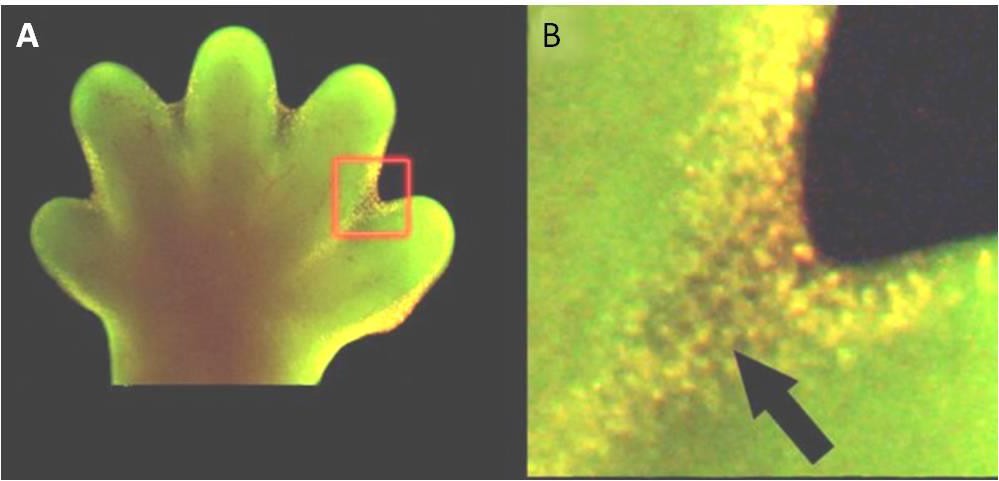
Apoptosis is also essential for normal embryological development. In vertebrates, for example, early stages of development include the formation of web-like tissue between individual fingers and toes (Figure 9.15). During the course of normal development, these unneeded cells must be eliminated, enabling fully separated fingers and toes to form. A cell signaling mechanism triggers apoptosis, which destroys the cells between the developing digits.
9.3.2 Termination of Signaling Pathways
The aberrant signaling often seen in tumor cells is proof that the termination of a signal at the appropriate time can be just as important as the initiation of a signal. One method of stopping a specific signal is to degrade the ligand or remove it so that it can no longer access its receptor. One reason that hydrophobic hormones like estrogen and testosterone trigger long-lasting events is because they bind to carrier proteins. These proteins allow the insoluble molecules to be soluble in blood, but they also protect the hormones from degradation by circulating enzymes.
Inside the cell, many different enzymes reverse the cellular modifications that result from signaling cascades. For example, phosphatases are enzymes that remove the phosphate group attached to proteins by kinases in a process called dephosphorylation. cAMP is degraded into AMP by phosphodiesterase, thereby terminating its signal. Similarly, the release of calcium stores is reversed by Ca2+ pumps that are located in cell membranes.

