12.6 Sediment Distribution
Now that we have an understanding of the types of sediments found in the ocean, we can turn our attention to the processes that cause different types of sediments to dominate in different locations. Sediment accumulation will depend on the the amount of material coming from the source, the distance from the source, the amount of time that sediment has had to accumulate, how well the sediments are preserved, and the amounts of other types of sediments that are also being added to the system.
Rates of sediment accumulation are relatively slow throughout most of the ocean, in many cases taking thousands of years for any significant deposits to form. Lithogenous sediment accumulates the fastest, on the order of 1 m or more per thousand years for coarser particles. However, sedimentation rates near the mouths of large rivers with high discharge can be orders of magnitude higher. Biogenous oozes accumulate at a rate of about 1 cm per thousand years, while small clay particles are deposited in the deep ocean at around 1 mm per thousand years. As described in section 12.4, manganese nodules have an incredibly slow rate of accumulation, gaining 0.001 mm per thousand years.
Marine sediments are thickest near the continental margins (refer to figure 12.1.1) where they can be over 10 km thick. This is because the crust near passive continental margins is often very old, allowing for a long period of accumulation, and because there is a large amount of terrigenous sediment input coming from the continents. Near mid-ocean ridge systems where new oceanic crust is being formed, sediments are thinner, as they have had less time to accumulate on the younger crust. As you move away from the ridge spreading center the sediments get progressively thicker (see section 4.5), increasing by approximately 100-200 m of sediment for every 1000 km distance from the ridge axis. With a seafloor spreading rate of about 20-40 km/million years, this represents a sediment accumulation rate of approximately 100-200 m every 25-50 million years.
Figure 12.6.1 shows the distribution of the major types of sediment on the ocean floor. Cosmogenous sediments could potentially end up in any part of the ocean, but they accumulate in such small abundances that they are overwhelmed by other sediment types and thus are not dominant in any location. Similarly, hydrogenous sediments can have high concentrations in specific locations, but these regions are very small on a global scale. So we will mostly ignore cosmogenous and hydrogenous sediments in the discussion of global sediment patterns.
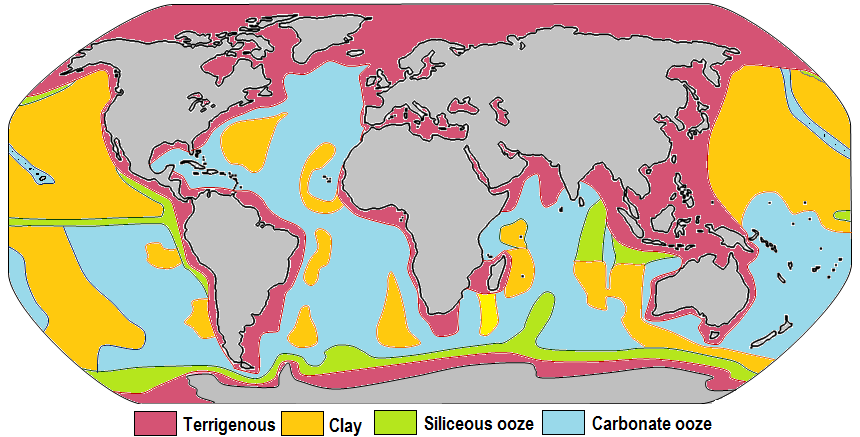
Coarse lithogenous/terrigenous sediments are dominant near the continental margins as runoff, river discharge, and other processes deposit vast amounts of these materials on the continental shelf (section 12.2). Much of this sediment remains on or near the shelf, while turbidity currents can transport material down the continental slope to the deep ocean floor. Lithogenous sediment is also common at the poles where thick ice cover can limit primary production, and glacial breakup deposits sediments along the ice edge. Coarse lithogenous sediments are less common in the central ocean, as these areas are too far from the sources for these sediments to accumulate. Very small clay particles are the exception, and as described below, they can accumulate in areas that other lithogenous sediment will not reach.
The distribution of biogenous sediments depends on their rates of production, dissolution, and dilution by other sediments. We learned in section 7.4 that coastal areas display very high primary production, so we might expect to see abundant biogenous deposits in these regions. However, recall that sediment must be >30% biogenous to be considered a biogenous ooze, and even in productive coastal areas there is so much lithogenous input that it swamps the biogenous materials, and that 30% threshold is not reached. So coastal areas remain dominated by lithogenous sediment, and biogenous sediments will be more abundant in pelagic environments where there is little lithogenous input.
In order for biogenous sediments to accumulate their rate of production must be greater than the rate at which the tests dissolve. Silica is undersaturated throughout the ocean and will dissolve in seawater, but it dissolves more readily in warmer water and lower pressures; in other words, it dissolves faster near the surface than in deep water. Silica sediments will therefore only accumulate in cooler regions of high productivity where they accumulate faster than they dissolve. This includes upwelling regions near the equator and at high latitudes where there are abundant nutrients and cooler water. Oozes formed near the equatorial regions are usually dominated by radiolarians, while diatoms are more common in the polar oozes. Once the silica tests have settled on the bottom and are covered by subsequent layers, they are no longer subject to dissolution and the sediment will accumulate. Approximately 15% of the seafloor is covered by siliceous oozes.
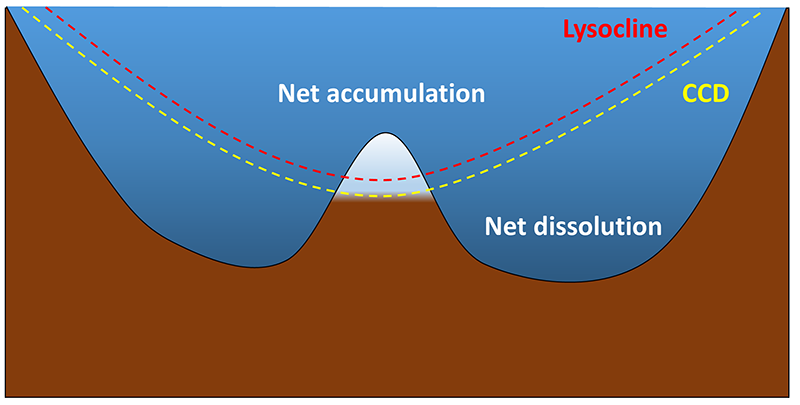
Biogenous calcium carbonate sediments also require production to exceed dissolution for sediments to accumulate, but the processes involved are a little different than for silica. Calcium carbonate dissolves more readily in more acidic water. Cold seawater contains more dissolved CO2 and is slightly more acidic than warmer water (section 5.5). Therefore calcium carbonate tests are more likely to dissolve in colder, deeper, polar water than in warmer, tropical, surface water. At the poles the water is uniformly cold, so calcium carbonate readily dissolves at all depths, and carbonate sediments do not accumulate. In temperate and tropical regions calcium carbonate dissolves more readily as it sinks into deeper water. The depth at which calcium carbonate dissolves as fast as it accumulates is called the calcium carbonate compensation depth, or calcite compensation depth, or simply the CCD. The lysocline represents the depths where the rate of calcium carbonate dissolution increases dramatically (similar to the thermocline and halocline). At depths shallower than the CCD carbonate accumulation will exceed the rate of dissolution, and carbonate sediments will be deposited. In areas deeper than the CCD, the rate of dissolution will exceed production, and no carbonate sediments can accumulate (Figure 12.6.2). The CCD is usually found at depths of 4 – 4.5 km, although it is much shallower at the poles where the surface water is cold. Thus calcareous oozes will mostly be found in tropical or temperate waters less than about 4 km deep, such as along the mid-ocean ridge systems and atop seamounts and plateaus. The CCD is deeper in the Atlantic than in the Pacific since the Pacific contains more CO2, making the water more acidic and calcium carbonate more soluble. This, along with the fact that the Pacific is deeper, means that the Atlantic contains more calcareous sediment than the Pacific. All told, about 48% of the seafloor is dominated by calcareous oozes.
Much of the rest of the deep ocean floor (about 38%) is dominated by abyssal clays. This is not so much a result of an abundance of clay formation, but rather the lack of any other types of sediment input. The clay particles are mostly of terrestrial origin, but because they are so small they are easily dispersed by wind and currents, and can reach areas inaccessible to other sediment types. Clays dominate in the central North Pacific, for example. This area is too far from land for coarse lithogenous sediment to reach, it is not productive enough for biogenous tests to accumulate, and it is too deep for calcareous materials to reach the bottom before dissolving. Because clay particles accumulate so slowly, the clay-dominated deep ocean floor is often home to hydrogenous sediments like manganese nodules. If any other type of sediment was produced here it would accumulate much more quickly and would bury the nodules before they had a chance to grow.
unconsolidated particles of mineral or rock that settle to the seafloor (12.1)
sediment derived from preexisting rock (12.2)
a sediment composed of >30% biogenous material (12.3)
sediment particle that is less than 1/256 mm in diameter (12.1)
spherical accumulations of manganese and other metals that form slowly through precipitation on the seafloor (12.4)
the region of transition from the land to the deep sea floor, i.e. between continental and oceanic crust (1.2)
the uppermost layer of the Earth, ranging in thickness from about 5 km (in the oceans) to over 50 km (on the continents) (3.2)
a boundary between a continent and an ocean at which there is no tectonic activity (e.g., the eastern edge of North America) (1.2)
referring to sedimentary particles that originated on a continent (12.2)
an underwater mountain system along divergent plate boundaries, formed by plate tectonics (4.5)
the Earth’s crust underlying the oceans (as opposed to continental crust) (3.2)
sediment derived from extraterrestrial sources (12.5)
sediments formed from the precipitation of dissolved substances (12.4)
flow of water down a slope, either across the ground surface, or within a series of channels (12.2)
the shallow (typically less than 200 m) and flat sub-marine extension of a continent (1.2)
a current moving down downhill along the bottom, driven by the weight of the sediment within it (1.2)
the steeper part of a continental margin, that slopes down from a continental shelf towards the abyssal plain (1.2)
the synthesis of organic compounds from aqueous carbon dioxide by plants, algae, and bacteria (7.1)
sediment created from the remains of organisms (12.3)
relating to the open ocean (1.3)
process by which deeper water is brought to the surface (9.5)
in the context of primary production, substances required by photosynthetic organisms to undergo growth and reproduction (5.6)
microscopic (0.1 to 0.2 mm) marine protozoa that produce silica shells (12.3)
photosynthetic algae that make their tests (shells) from silica (7.2)
the shell-like hard parts (either silica or carbonate) of small organisms such as radiolarians and foraminifera (12.3)
the depth in the ocean (typically around 4000 m) below which carbonate minerals are soluble (12.6)
the depths where the rate of calcium carbonate dissolution increases dramatically over surface waters (12.6)
a region in the water column where there is a dramatic change in temperature over a small change in depth (6.2)
where there is a dramatic change in salinity over a small change in depth (5.3)
a submerged mountain rising from the seafloor (4.9)

